
KB PURE is proud to present the results of the clinical trials, conducted in 2021 by an independent test institute in the EU. After 5 years of research and treatment experience with our formulas these studies have proven what we stand for – various pathologies can be treated even without pharmaceutical ingredients, using our skin care.
Skin rejuvenation starts with with derivatives of the specific acids in a balanced acidity environment without compromising the integrity of the skin barrier.
Clarifying skin blemishes is caused with ingredients that inhibit the enzyme tyrosinase in melanin cells without the use of RNA blockers that can harm the skins health.
Balance for problematic skin is treated with organic molecules that reduce comedogenic inflammatory processes that impair the aesthetic integrity of the skin.
Treatment of a variety of different pathologies (rosacea, psoriasis, atopic dermatitis, etc ..) is done with a combination of provided products. Various plant-based anti-inflammatory inhibitors are safe to use and do not cause dependency.
The use of the preparations and their adjustment is done after a diagnosis by trained skin therapists (esthetician’s and dermatologists).
The clinical trials from Institute Hamilton in Poland were conducted under the supervision of dermatologists on 90 volunteers.
KB PURE is proud to present the results of the clinical trials, conducted in 2021 by an independent test institute in the EU. After 5 years of research and treatment experience with our formulas these studies have proven what we stand for – various pathologies can be treated even without pharmaceutical ingredients, using our skin care.
Skin rejuvenation starts with with derivatives of the specific acids in a balanced acidity environment without compromising the integrity of the skin barrier.
Clarifying skin blemishes is caused with ingredients that inhibit the enzyme tyrosinase in melanin cells without the use of RNA blockers that can harm the skins health.
Balance for problematic skin is treated with organic molecules that reduce comedogenic inflammatory processes that impair the aesthetic integrity of the skin.
Treatment of a variety of different pathologies (rosacea, psoriasis, atopic dermatitis, etc ..) is done with a combination of provided products. Various plant-based anti-inflammatory inhibitors are safe to use and do not cause dependency.
The use of the preparations and their adjustment is done after a diagnosis by trained skin therapists (esthetician’s and dermatologists).
The clinical trials from Institute Hamilton in Poland were conducted under the supervision of dermatologists on 90 volunteers.
JS HAMILTON PROVIDES LABORATORY INSPECTION AND TESTING SERVICES FOR PHARMACEUTICALS AND COSMETICS IN POLAND
KB-Pure’s official clinical studies result at Hamilton – Acne Vulgaris

Tested Products: Pure Moisturizing Cream, Azilaccutane Cream, An-bscess
Tested Products: Pure Moisturizing Cream, Azilaccutane Cream, An-bscess
JS HAMILTON PROVIDES LABORATORY INSPECTION AND TESTING SERVICES FOR PHARMACEUTICALS AND COSMETICS
IN POLAND
KB-Pure’s official clinical studies result at Hamilton – Acne Vulgaris

Tested Products: Pure Moisturizing Cream,
Azilaccutane Cream, An-bscess

Tested Products: Pure Moisturizing Cream,
Azilaccutane Cream, An-bscess
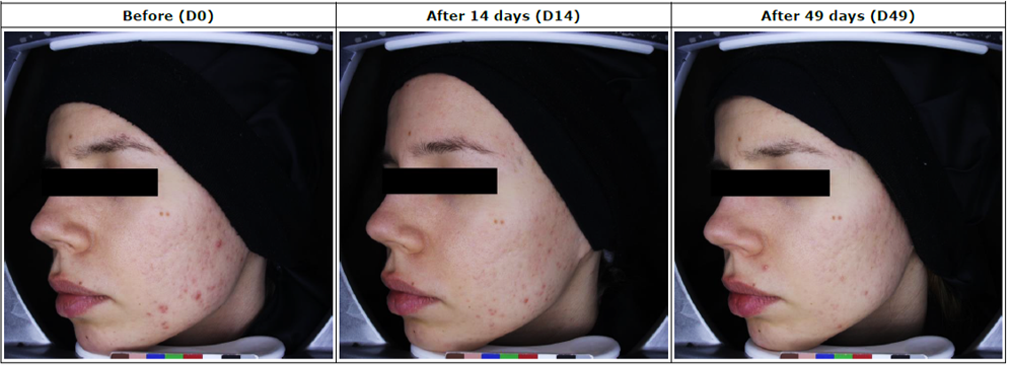
Assessment of skin condition in 30 acne vulgaris patients, following treatment with Pure Moisturizing Cream, Azilaccutane Cream, An-Bscess for 14 & 49 days.
Hamilton’s Conclusions:
Reduces Redness of inflammatory spots in 19% after 14 days in 90% of patients and 41% after 49 days in 97% of patients.
Reduces skin redness in 5% after 14 days in 75% of patients and 12% after 49 days in 97% of patients.
– Instrumental test of level of erythema / redness of the skin (hemoglobin content) by using Mexameter® MX 18.
– Instrumental test of sebum level by using Sebumeter® SM 815.
Patients who showed improvement in the symptoms of Acne Voulgaris after 14 & 49 days of treatment
Assessment of skin condition in 30 Acne Vulgaris patients, following treatment with Pure Moisturizing Cream, Azilaccutane Cream, An-bsces.
Hamilton’s Conclusions:
Reduces Redness of inflammatory spots in 19% after 14 days in 90% of patients and 41% after 49 days in 97% of patients.
Reduces skin redness in 5% after 14 days in 75% of patients and 12% after 49 days in 97% of patients.
– Instrumental test of level of erythema/redness of the skin (hemoglobin content) by using Mexameter® MX 18.
– Instrumental test of sebum level by using Sebumeter® SM 815.

Patients who showed improvement in the symptoms
of Acne Voulgaris after 14 & 49 days of treatment
The results obtained in the test allow to conclude, that the product used as intended is moderately tolerated by the people, in whom there is not a contraindication to its use.
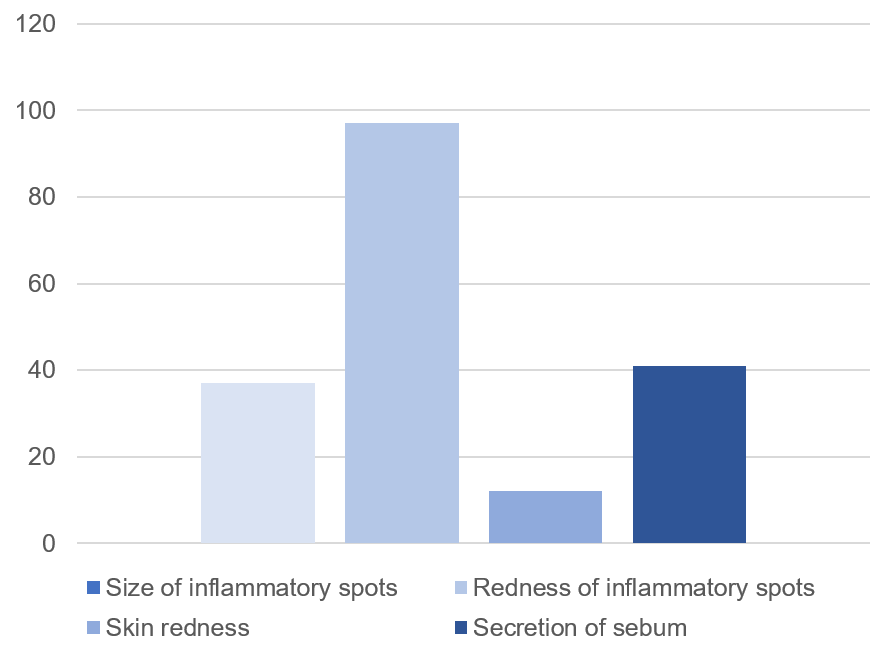
Reduces the diminution of the size of inflammatory spots.
Reduces the diminution of the redness of inflammatory spots.
Reduces skin redness.
Reduces secretion of sebum.
The results obtained in the test allow to conclude, that the product used as intended is moderately tolerated by the people, in whom there is not a contraindication to its use.
Reduces the diminution of the size of inflammatory spots.
Reduces the diminution of the redness of inflammatory spots.
Reduces skin redness.
Reduces secretion of sebum.
JS HAMILTON PROVIDES LABORATORY INSPECTION AND TESTING SERVICES FOR PHARMACEUTICALS AND COSMETICS IN POLAND
KB-Pure’s official clinical studies result at Hamilton – Atopic Dermatitis
Tested Products: Tested Products: Revive Serum, Relaxer Cream, Relaxer Spray
Tested Products: Tested Products: Revive Serum, Relaxer Cream, Relaxer Spray
JS HAMILTON PROVIDES LABORATORY INSPECTION AND TESTING SERVICES FOR PHARMACEUTICALS AND COSMETICS IN POLAND
KB-Pure’s official clinical studies result at Hamilton – Atopic Dermatitis

Tested Products: Tested Products: Revive
Serum, Relaxer Cream, Relaxer Spray

Tested Products: Tested Products: Revive Serum, Relaxer Cream, Relaxer Spray
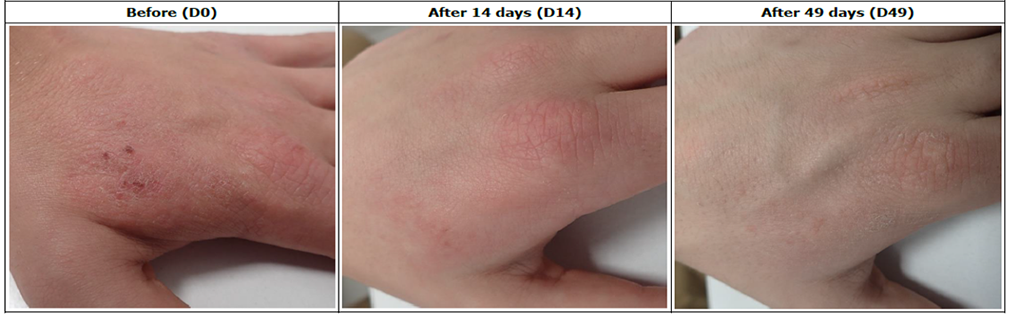
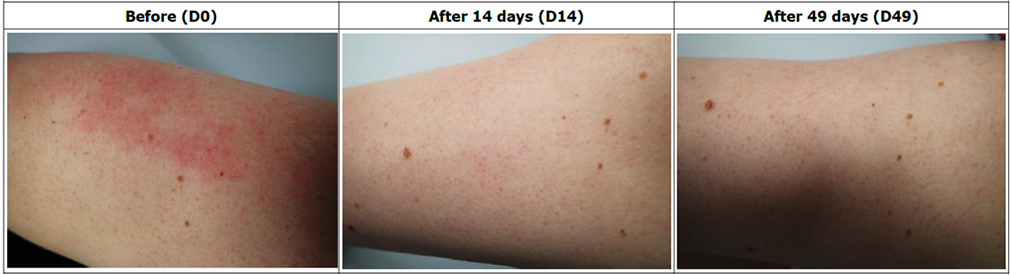
Assessment of skin condition in 30 Atopic Dermatitis patients, following treatment with REVIVE SERUM, RELAXER CREAM, RELAXER SPRAY for 49 days.
Hamilton’s Conclusions:
Reduces skin itching feeling in 83% of patients.
Decreases TEWL in 16% after 14 days and 22% after 49 days in 93% of patients.
Moisturizes the skin in 11% after 14 days and 22% after 49 days in 93% of patients.
Reinforces the hydro-lipid barrier of the skin in 16% after 14 days and 22% after 49 days in 83% of patients.
Efficacy is confirmed in 46% after 14 days in 72% of patients and 80% after 49 days in 83% of patients.
– Measurement of face skin moisturization was taken by Corneometer.
– Measurement of skin trans epidermal water loss was taken by Tewameter.
– Photography of the full face view using Visioface® RD or photography taken using Camera for the zone other than face.
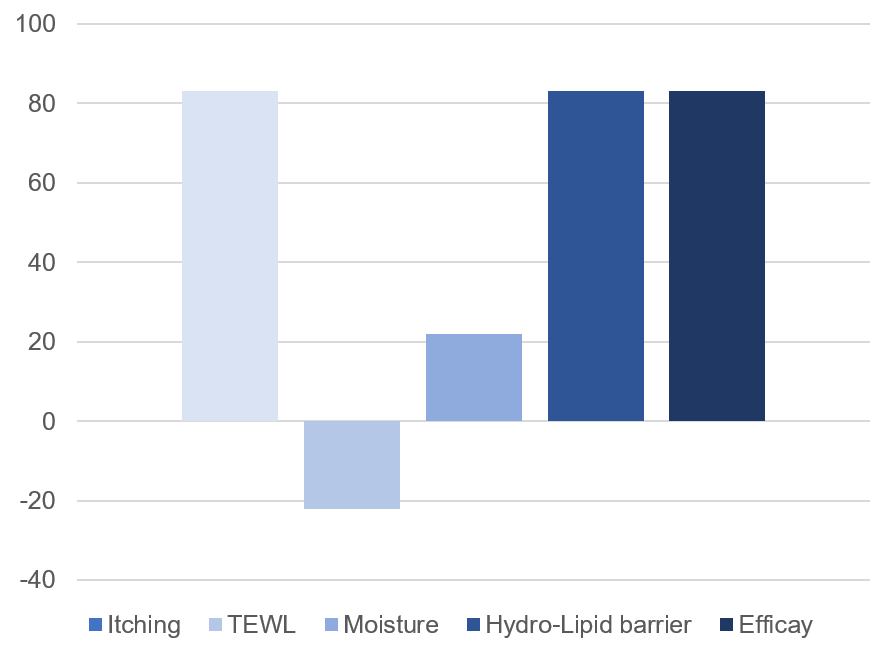
Patients who showed improvement in the symptoms of atopic dermatitis after 49 days of treatment
Assessment of skin condition in 30 Acne Vulgaris patients, following treatment with Pure Moisturizing Cream, Azilaccutane Cream, An-bsces.
Hamilton’s Conclusions:
Reduces skin itching feeling in 83% of patients.
Decreases TEWL in 16% after 14 days and 22% after 49 days in 93% of patients.
Moisturizes the skin in 11% after 14 days and 22% after 49 days in 93% of patients.
Reinforces the hydro-lipid barrier of the skin in 16% after 14 days and 22% after 49 days in 83% of patients.
Efficacy is confirmed in 46% after 14 days in 72% of patients and 80% after 49 days in 83% of patients.
– Measurement of face skin moisturization was taken by Corneometer.
– Measurement of skin trans epidermal water loss was taken by Tewameter.
– Photography of the full face view using Visioface® RD or photography taken using Camera for the zone other than face.

Patients who showed improvement in the symptoms of
atopic dermatitis after 49 days of treatment
No adverse events were observed.
Significant improvement was shown in atopic dermatitis symptoms.
No patients required additional steroidal treatment.
No cases of worsening condition of atopic dermatitis were recorded.
No secondary infections were observed.
No adverse events were observed.
Significant improvement was shown in atopic dermatitis symptoms.
No patients required additional steroidal treatment.
No cases of worsening condition of atopic dermatitis were recorded.
No secondary infections were observed.
JS HAMILTON PROVIDES LABORATORY INSPECTION AND TESTING SERVICES FOR PHARMACEUTICALS AND COSMETICS IN POLAND
KB-Pure’s official clinical studies result at Hamilton – Pigmentation & Whitening
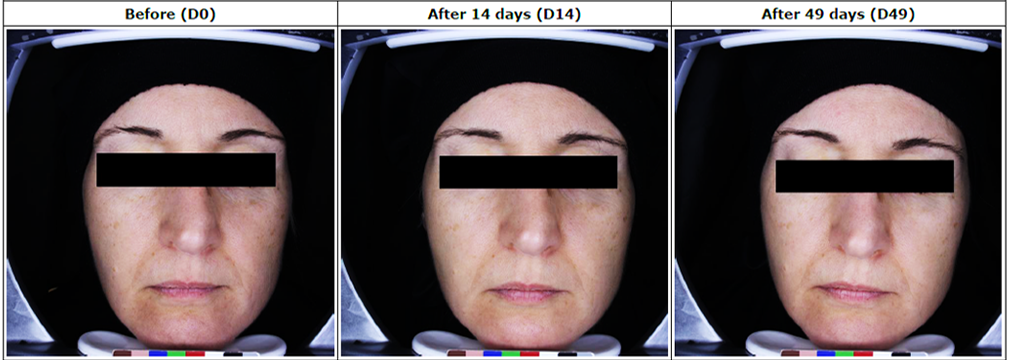
Tested Products: Antirossine Booster Serum, Phyto Whitening Cream, Ultra Protector, Simply Pure For Oily Skin/Dry Skin
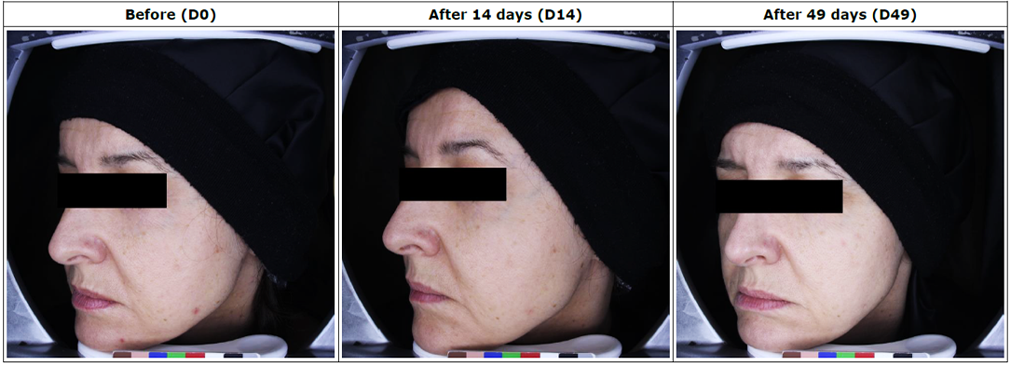
Tested Products: Antirossine Booster Serum, Phyto Whitening Cream, Ultra Protector, Simply Pure For Oily Skin/Dry Skin
JS HAMILTON PROVIDES LABORATORY INSPECTION AND TESTING SERVICES FOR PHARMACEUTICALS AND COSMETICS IN POLAND
KB-Pure’s official clinical studies result at Hamilton – Pigmentation & Whitening

Tested Products: Antirossine Booster Serum, Phyto Whitening Cream, Ultra Protector, Simply Pure For Oily Skin/Dry Skin

Tested Products: Antirossine Booster Serum, Phyto Whitening Cream, Ultra Protector, Simply Pure For Oily Skin/Dry Skin
Assessment of skin condition in 30 Pigmentation & Whitening patients, following treatment with Antirossine Booster Serum, Phyto Whitening Cream, Ultra Protector, Simply Pure For Oily Skin/Dry Skin for 49 days.
Hamilton’s conclusions:
• Lightens the skin in 6% after 14 days in 100% of patients.
• Lightens the skin in 15% after 49 days in 100% of patients.
– The measurements of the studied zone: o Measurement of melanin level in the skin was taken by Mexameter® MX 18, The results of the intensity of the color of the skin (melanin level) measurements before application (D0), after 14 days (D14) and after 49 days (D49) of regular application in m. u.
– Photography of the full face view using Visioface® RD.
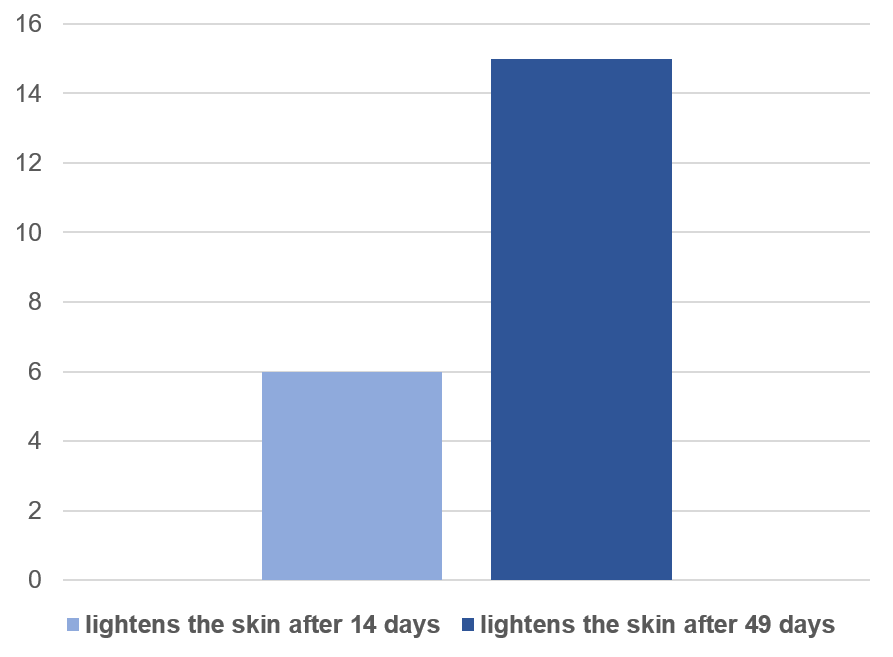
Patients who showed improvement in Pigmentation and
Whitening after 14 & 49 days of treatment

Patients who showed improvement in Pigmentation and Whitening after 14 & 49 days of treatment
Assessment of skin condition in 30 Pigmentation & Whitening patients, following treatment with Antirossine Booster Serum, Phyto Whitening Cream, Ultra Protector, Simply Pure For Oily Skin/Dry Skin for 49 days.
Hamilton’s conclusions:
• Lightens the skin in 6% after 14 days in 100% of patients.
• Lightens the skin in 15% after 49 days in 100% of patients.
– The measurements of the studied zone: o Measurement of melanin level in the skin was taken by Mexameter® MX 18, The results of the intensity of the color of the skin (melanin level) measurements before application (D0), after 14 days (D14) and after 49 days (D49) of regular application in m. u.
– Photography of the full face view using Visioface® RD.

Patients who showed improvement in Pigmentation and
Whitening after 14 & 49 days of treatment
The results obtained in the test allow concluding, that the products used as intended are very well tolerated by the people, in whom there is not a contraindication to its use.
The products lighten the skin Lightens the skin after 14 days and after 49 days of regular use.
There were no negative symptoms and feelings that might indicate intolerance to any component of the product, such as irritation, burning sensation, redness, or itching.
Did not cause dryness at the site of application in any subjects.
The results obtained in the test allow concluding, that the products used as intended are very well tolerated by the people, in whom there is not a contraindication to its use.
The products lighten the skin Lightens the skin after 14 days and after 49 days of regular use.
There were no negative symptoms and feelings that might indicate intolerance to any component of the product, such as irritation, burning sensation, redness, or itching.
Did not cause dryness at the site of application in any subjects.
Home Use & Complimentary
The perfect treatment for age signs
Rehabilitation and skin relaxation
Problematic skin care / acne
Maintaining a youthful skin look
Lightening of skin spots and pigmentation
Treatment Cart